
Quantitative Whole Body Autoradiography (QWBA)
Quantitative Whole Body Autoradiography (QWBA) studies represent a critical part of a comprehensive preclinical ADME strategy. Quantifying distribution of radiolabeled drug to various target and non-target tissues, measuring rates of elimination, and generating key dosimetry calculations preceding clinical trials are all important for drug development.
QWBA can provide dosimetric calculations before human radiolabeled studies and can help evaluate drug interactions by observing changes in tissue distribution of a compound when co-administered with another drug. Investigation of a compound’s affinity with the site of pharmacological action is vital for preclinical safety and toxicology assessments.
Distribution & Dosimetry Data
Results from a QWBA study (in addition to data from a rodent mass balance study) provide critical exposure inputs for dosimetry calculations, which are required for planning radiolabeled mass balance studies in human absorption, metabolism, and excretion (hAME) studies. FDA and other agencies mandate dosimetry (exposure) calculations to provide “reasonable prediction of the radiation absorbed by human volunteers”1 to address regulatory requirements that radioactivity from the radiolabeled drug will not harm human subjects2. Data from subsequent human mass balance and other in vitro/in vivo ADME studies are required for filing a New Drug Application (NDA) to get a drug approved for market.
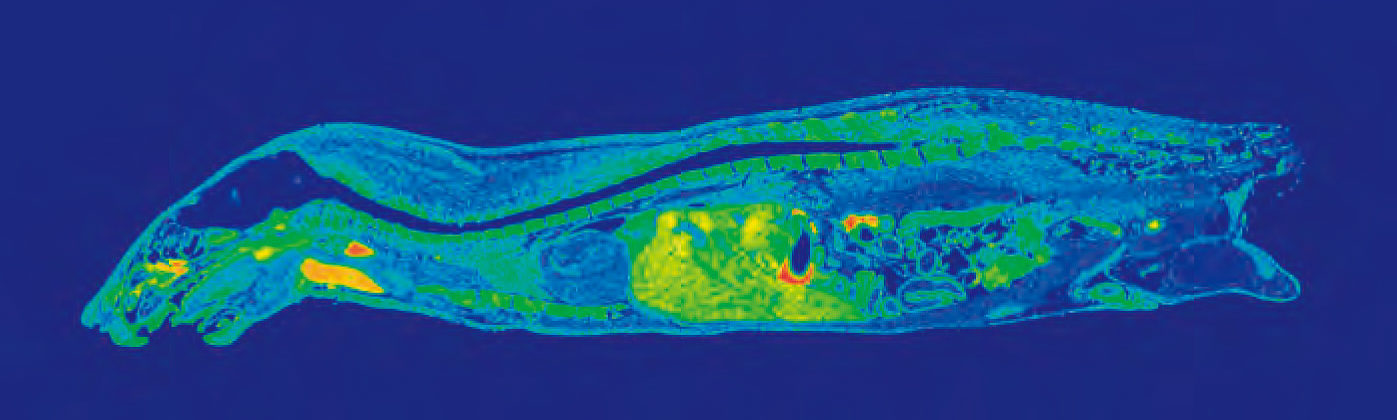
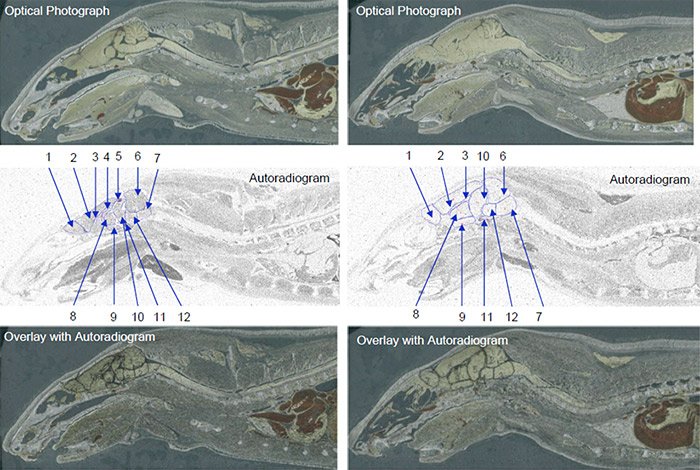
Data obtained from quantitative whole body autoradiography studies are also applicable to other important PK considerations, i.e. distribution. A QWBA study can tell you how a compound is distributed to both the targeted and non-targeted tissues as well as accumulation in specific parts of an organ (for example, substructures in the brain as pictured in Figure 1).
Our Approach to QWBA
Whole body autoradiography is unique in its ability to give a drug developer a whole picture of how their drug travels through the body over time. Using images generated by a QWBA study, you can observe clear localization of radioactivity in tissues and organs to demonstrate distribution at continual time points, quantify radioactivity in targeted tissue to estimate effective dose, and detect radioactivity in unexpected tissues.
Performing QWBA equips you with key pharmacokinetic data:
- Tissue distribution
- Dosimetry report
- Melanin binding
- Brain and cerebrospinal fluid exposure
- Estimation of elimination rate in various tissues
- Tumor penetration
- Detection of potential sites of drug accumulation
In a typical study package for QWBA, both albino and pigmented rats are used. Pigmented rats, such as Long-Evans, are necessary for the dosimetry calculation inputs, while albino rats such as Sprague-Dawley are selected to match the animals used in toxicity studies. By including animals of the tox species as well, e.g. Sprague-Dawley rats or mice, distribution data can be more closely correlated to earlier toxicity data.
Techniques involved in a Quantitative Whole Body Autoradiography study are highly specialized, and require many hours of experience to prepare slices correctly. Our team at the Drug Development Solutions Center in Tokai has expertise culminated from more than 50 years in the radioisotope industry. They have the capabilities to work with not only 14C- and 3H-labeled compounds but other isotopes as well to suit peptides, nucleotides, antibodies, and more for in vivo ADME studies. They can also cater to unusual routes of administration, and because their facility is equipped for many different in vivo study types, packages of multiple in vivo studies can be developed to run concurrently all in the same lab to get you consistent, high-quality data faster.
| Routes of Administration | Radionuclides | Animal Species |
|---|---|---|
| Oral Intravenous (administration or infusion) Percutaneous Subcutaneous Intramuscular Intracheal Opthalmic Colorectal Intraduodenal Nasal Intraocular Sublingual Intrauterine Intracerebral Intravesical Knee Joint | 14C 3H 125I 33P 35S 51Cr 111In 55,59Fe 65Zn 75Se 90Y 153Gd | Rat Mouse (including chimeric with humanized liver) Rabbit Dog Monkey Animal models of human disease (knockout models) |
Related Services:
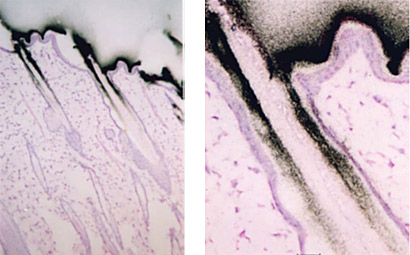
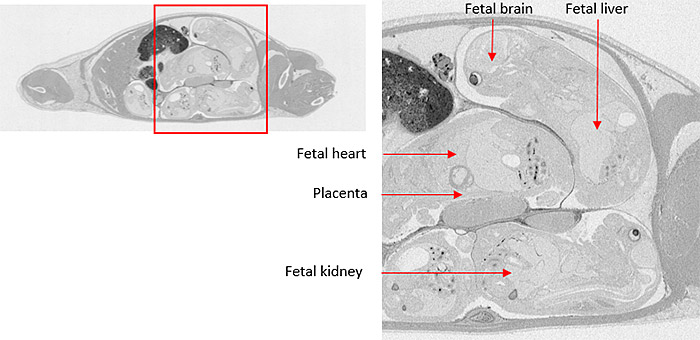
Microautoradiography (mARG) is also available to look more closely at distribution within tissues (Figure 2). Tissues available for mARG include liver, kidney, intestine, tumor, pancreas, skeletal muscle, spleen, retina adrenal gland, and thyroid gland. We also offer options for inclusion of pregnant animals for placental transfer data (seen in Figure 3).
References
- “CFR – Code of Federal Regulations Title 21.” Accessdata.fda.gov, Apr. 2019, www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=361.1
- Solon, Eric G. “Quantitative Whole Body Autoradiography (QWBA) and Imaging Mass Spectrometry (IMS): Can IMS Replace QWBA to Support Regulated Drug Discovery and Development.” Bioanalysis Zone, Bioanalysis Zone, 21 Mar. 2018, www.bioanalysis-zone.com/2018/03/09/quantitative-whole-body-autoradiography-qwba-imaging-mass-spectrometry-ims-can-ims-replace-qwba-support-regulated-drug-discovery-development/.

